Service Quality Complaint Hotline:021-62961702
Service Quality Complaint Hotline:021-62961702
28
2022
-
06
Over a hundred products entered the innovation/priority approval channel in the first half of 2022 (with a list attached)
For enterprises, entering the special approval process for innovative medical devices means that the product's time to market will be greatly shortened, while promoting the promotion and application of new medical device technologies; On the other hand, it is also a manifestation of the innovation ability of enterprises, which has attracted much attention from investors.
Author:
In recent years, the medical device review and approval system has been continuously reformed. In order to ensure the safety and effectiveness of medical devices, and encourage research and innovation of medical devices, the National Medical Products Administration issued a newly revised "Special Review Procedure for Innovative Medical Devices" on November 2, 2018, which has played a positive role in the innovative development of the medical device industry. At the same time, in order to ensure the clinical use needs of medical devices, the former State Food and Drug Administration issued the "Priority Approval Procedure for Medical Devices" on October 26, 2016, which played a huge role in improving people's health and hygiene levels.
For enterprises, entering the special approval process for innovative medical devices means that the product's time to market will be greatly shortened, while promoting the promotion and application of new medical device technologies; On the other hand, it is also a manifestation of the innovation ability of enterprises, which has attracted much attention from investors.
Overall
According to JOINCHAIN, in the first half of 2022, a total of 65 products from 55 enterprises entered the special approval channel for innovative medical devices, of which 36 products entered the national innovation approval channel and 29 products entered the provincial innovation approval channel; A total of 50 products from 30 enterprises have entered the priority approval channel, of which 3 products have entered the national priority approval channel and 47 products have entered the provincial priority approval channel. (Product list can be found in Appendix 1)
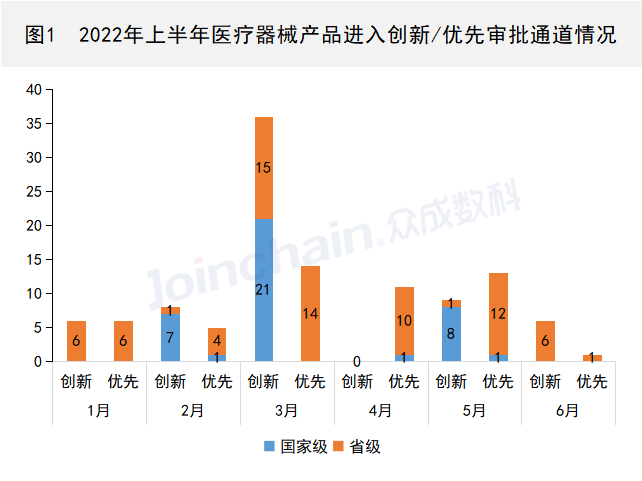
Data source: JOINCHAIN ® Zhongcheng Mathematics
key word:
RELATED NEWS